- Shanghai Zhongshen International Trade Co., Ltd. - Two decades of trade agency expertise.
- Service Hotline: 139 1787 2118
With the increasing prosperity of global trade,Cosmetics & Personal CareManufacturers and exporters are more frequently expanding their target markets overseas. The United States, as one of the largest economies in the world, is undoubtedly a very attractive market. However, entering the U.S. market is not easy and requires strict compliance with U.S. cosmetic regulations. The goal of this article is to provide a comprehensive guide for those who wish to export cosmetics to the United States.
First, we need to understandThe definition of cosmetics under the U.S. Federal Food, Drug, and Cosmetic Act:Articles intended to be rubbed, poured, sprinkled, or otherwise applied to the human body for cleansing, beautifying, promoting attractiveness, or altering the appearance. Common cosmetics include moisturizers, perfumes, lipsticks, nail polishes, eye and facial makeup, shampoos, permanent waves, hair colors, toothpastes, deodorants, etc.
For exporting cosmetics to the United States, it is essential to be familiar with the following regulatory requirements:
a. Prohibited and restricted substances:The 9 substances listed in 21 CFR 700.11-21 CFR 700.35 are prohibited in cosmetics. The use of colorants is also strictly limited, allowing only those listed in 21 CFR Part 73, 74, and 82, with attention to appropriate application areas.
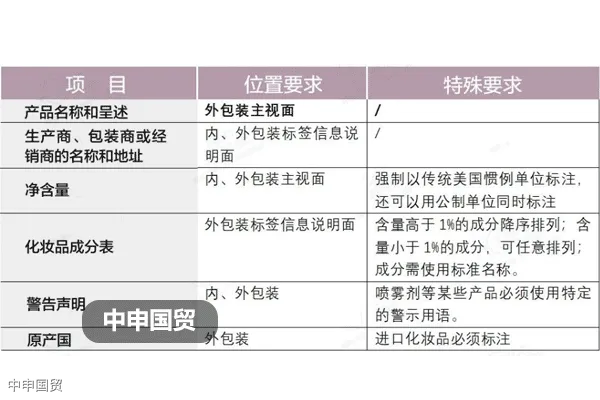
b. Labeling requirements:The products sales packaging must clearly and legibly display the product name, net quantity, company name and address, ingredient identification, warning statements, and safe usage instructions, among other information.
c. Safety assessment:Before sale, the product and its ingredients must undergo a thorough safety assessment.
d. Special requirements in California:For products sold in California, additional requirements under Proposition 65 must be met, including restrictions on substances such as lead, Cocamide DEA, and 1,4-Dioxane.
eGood Manufacturing Practices:Exporters also need to comply with Cosmetic Good Manufacturing Practices (GMP), which involve equipment, personnel, raw materials, production processes, laboratory management, records, labeling, and complaints, among other aspects.
In addition, some products may simultaneously meet the definitions of both cosmetics and drugs, such as anti-dandruff shampoo, fluoride toothpaste, antiperspirants, etc. These products need to comply with both cosmetic and pharmaceutical regulatory requirements. These regulations involve aspects such as approval, registration, good manufacturing practices, and labeling.
Overall, exporting cosmetics to the United States is a process that requires careful research and preparation, involving quite complex regulations and requirements. The above information is not legal advice and should not be considered as or represent the legal opinion or conclusion of government regulatory agencies.
For more information on cosmetic regulations, please visit the following websites:
U.S. FDA Cosmetic Regulations Website
U.S. Cosmetic Labeling Regulations
How to determine whether a product is a cosmetic, a drug, or both?
Special considerations for U.S. cosmetic regulation
| Product Range | U.S. Regulatory Categories |
|---|---|
| Anti-dandruff shampoo | Over-the-counter drugs (OTC) |
| Acne treatment products | Pharmaceuticals |
| Hair growth products | Pharmaceuticals |
| Sunscreen products | Over-the-counter drugs (OTC) |
| Cleansing wipes | Cosmetics & Personal Care |
| Temporary tattoos | Cosmetics & Personal Care |
| Soap | Cosmetics/General consumer products (depending on composition and function) |
| Plastic artificial nail tips | Cosmetics & Personal Care |
Related Recommendations
? 2025. All Rights Reserved. Shanghai ICP No. 2023007705-2  PSB Record: Shanghai No.31011502009912
PSB Record: Shanghai No.31011502009912